Environmental Challenges of Pharmaceuticals: Persistence
and Accumulation in Ecosystems
Published: November 2024
Download PDF
EnviroMail_21_Europe_ Environmental Challenges of Pharmaceuticals: Persistence and Accumulation in Ecosystems

The drugs are used extensively not only in human and veterinary medicine but are also widely found in livestock feed. Because pharmaceuticals are designed to be effective even at low concentrations, these specific contaminants can exist in the environment at extremely low levels. As with pesticides, these compounds can accumulate in different parts of the ecosystem. Since conventional wastewater treatment plants cannot adequately remove these substances, they are released into the environment, including surface and groundwater, and consequently into drinking water sources.
Pharmaceuticals as "New" Pesticides...
Pharmaceuticals represent a broad and chemically diverse group of substances that is continuously expanding and is characterised by a wide range of clinical effects and extensive use, often leading to overuse, in both human and veterinary medicine. From an environmental perspective, they are classified as organic micropollutants due to their presence in water at low concentrations (ranging from ng/L to μg/L). Both the public and experts are concerned not only about their negative impacts on natural ecosystems but also about their presence in drinking water and the related effects on human health. Their contribution to the emergence of antimicrobial resistance (AMR) is also significant, as it greatly complicates the treatment of infectious diseases and represents a major challenge for current and future medicine.
Drugs can enter the environment through various pathways. One of the primary sources of contamination is wastewater from households and health and social care facilities, which contains human urine and faeces, as well as unused or expired pharmaceuticals. Another significant source is wastewater from the pharmaceutical industry. Consequently, wastewater treatment plants (WWTPs) themselves become sources of environmental contamination, as they are unable to effectively remove pharmaceuticals, allowing them to be transported further into the environment [1].
This occurs through treated wastewater discharged into surface waters and sewage sludge used as fertiliser. Livestock production also contributes significantly, as animals excrete pharmaceuticals in their urine and faeces, leading to contamination via aquaculture, grazing, and manure application on agricultural land. Pharmaceuticals enter the environment in both unchanged and metabolised forms; the latter results from the body's conversion of substances into more mobile polar forms.
European Legislation
EU legislation governing the quality of various types of water is gradually incorporating pharmaceuticals into its relevant documents at both European and regional levels. Regarding municipal wastewater, a proposal for a revised directive on wastewater treatment has been introduced, and the legislative process is nearly complete. Member States will now be required to ensure both the monitoring of antimicrobial resistance (for agglomerations with populations of 100,000 or more) and the removal of the widest possible range of micropollutants, particularly pharmaceuticals (by the end of 2045 for all wastewater treatment plants with a load of 150,000 or more). To determine whether the required minimum removal rate of 80% has been met, 12 parameters will need to be monitored, almost all of which are pharmaceuticals. The list of the relevant compounds is summarised in Table 1.

Pharmaceuticals at WWTPs
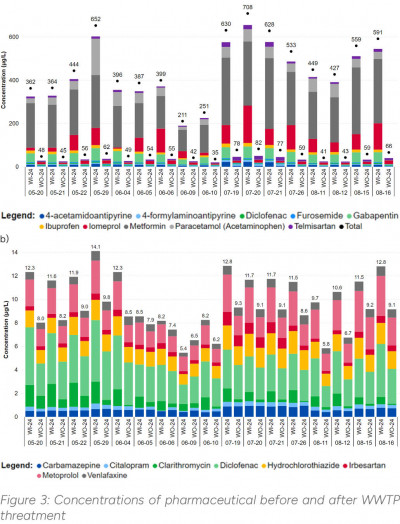
ALS laboratory research projects have examined the rate of pharmaceuticals removal through conventional mechanical-biological treatment of wastewater as conducted at standard municipal WWTP. The results are shown in the graphs in Figure 3. Figure 3 (a) presents the results for the analytes most frequently detected in wastewater and at the highest concentrations, their removal rate is as high as 80-90%. Figure 3 (b) illustrates the pharmaceuticals proposed for monitoring under the mentioned EU regulations. It is evident that the removal rate for these analytes is lower, averaging only about 20%, highlighting the importance of their monitoring.
ALS Laboratories have developed and accredited a multiresidue methods for the determination of more than 100 different pharmaceuticals and their metabolites in various types of water. All of our analytical methods use the LC/MS/MS technique for analytes determination, which provides high sensitivity, selectivity and precision of measurement and allows the determination of target compounds at the very low limits required for residue analysis.
References:
[1] DOI: 10.1016/j.jhazmat.2009.10.100
[2] European Parliament legislative resolution: Document P9_TA(2024)0222
[3] Proposal for a Directive of the European Parliament and of the Council: Document 52022PC0540
[4] Commission Implementing Decision (EU) 2022/679: Document 32022D0679


